- Case report
- Open access
- Published:
Transformation into acute myeloid leukemia with t(8;21)(q22;q22.1); RUNX1::RUNX1T1 from JAK2-mutated essential thrombocythemia: a case report
Journal of Medical Case Reports volume 18, Article number: 372 (2024)
Abstract
Background
Blast transformation is a rare but well-recognized event in Philadelphia-negative myeloproliferative neoplasms associated with a poor prognosis. Secondary acute myeloid leukemias evolving from myeloproliferative neoplasms are characterized by a unique set of cytogenetic and molecular features distinct from de novo disease. t(8;21) (q22;q22.1); RUNX1::RUNX1T1, one of the most frequent cytogenetic abnormalities in de novo acute myeloid leukemia, is rarely observed in post-myeloproliferative neoplasm acute myeloid leukemia. Here we report a case of secondary acute myeloid leukemia with t(8;21) evolving from JAK2-mutated essential thrombocythemia.
Case presentation
The patient was a 74-year-old Japanese woman who was referred because of thrombocytosis (platelets 1046 × 109/L). Bone marrow was hypercellular with increase of megakaryocytes. Chromosomal analysis presented normal karyotype and genetic test revealed JAK2 V617F mutation. She was diagnosed with essential thrombocythemia. Thrombocytosis had been well controlled by oral administration of hydroxyurea; 2 years after the initial diagnosis with ET, she presented with leukocytosis (white blood cells 14.0 × 109/L with 82% of blasts), anemia (hemoglobin 91 g/L), and thrombocytopenia (platelets 24 × 109/L). Bone marrow was hypercellular and filled with 80% of myeloperoxidase-positive blasts bearing Auer rods. Chromosomal analysis revealed t(8;21) (q22;q22.1) and flow cytometry presented positivity of CD 13, 19, 34, and 56. Molecular analysis showed the coexistence of RUNX1::RUNX1T1 chimeric transcript and heterozygous JAK2 V617F mutation in leukemic blasts. She was diagnosed with secondary acute myeloid leukemia with t(8;21)(q22;q22.1); RUNX1::RUNX1T1 evolving from essential thrombocythemia. She was treated with combination chemotherapy with venetoclax and azacytidine. After the first cycle of the therapy, blasts disappeared from peripheral blood and decreased to 1.4% in bone marrow. After the chemotherapy, RUNX1::RUNX1T1 chimeric transcript disappeared, whereas mutation of JAK2 V617F was still present in peripheral leukocytes.
Conclusions
To our best knowledge, the present case is the first one with JAK2 mutation preceding the acquisition of t(8;21). Our result suggests that t(8;21); RUNX1::RUNX1T1 can be generated as a late event in the progression of JAK2-mutated myeloproliferative neoplasms. The case presented typical morphological and immunophenotypic features associated with t(8;21) acute myeloid leukemia.
Background
Philadelphia (Ph)-negative myeloproliferative neoplasms (MPNs) are clonal hematopoietic disorders characterized by the proliferation of mature blood cells. Among them, polycythemia vera and essential thrombocythemia (ET) are the most frequent entities associated with chronic and indolent clinical course. Activating mutations of the JAK/STAT signal pathway, primarily JAK2, MPL, and CALR, are present in the great majority of patients, exerting as primary pathogenesis leading to the proliferation of mature blood cells [1].
Blast transformation is a rare but well-recognized event in Ph-negative MPNs, associated with a grim prognosis. Secondary acute myeloid leukemias (AMLs) evolving from MPNs are characterized by a unique set of cytogenetic and molecular features distinct from de novo AML [2,3,4,5]. Somatic mutations in TP53, ASXL1, IDH1/2, TET2, and RUNX1 genes were reported to occur frequently in post-MPN AML, comparing with those in de novo disease [3,4,5]. On the contrary, structural alterations of chromosomes such as t(8;21)(q22;q22.1), inv(16)(p13.1q22)/t(16;16)(p13.1;q22), and t(15;17)(q22;q11-12), known as the most common cytogenetic aberrations in de novo AML, are quite rare in post-MPN AML [6,7,8,9,10,11,12,13,14,15,16]. Here we report a case of t(8;21) AML evolved from JAK2-mutated ET.
Case presentation
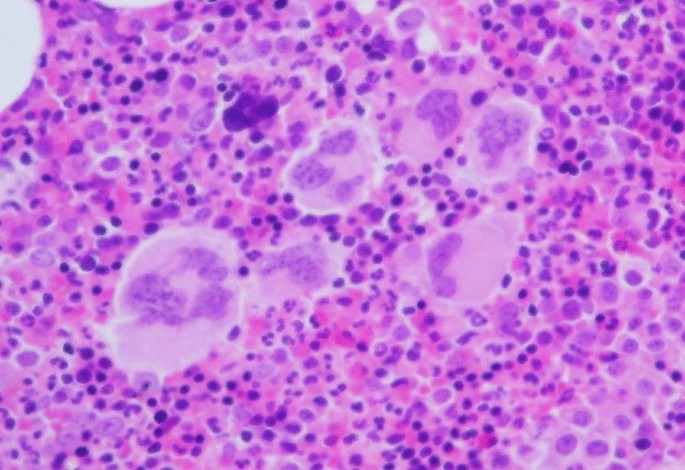
A 72-year-old Japanese female patient was referred to the hospital because of thrombocytosis. She had no significant medical history. Peripheral blood showed hemoglobin 132 g/L, platelets 1046 × 109/L, and white blood cells 8.4 × 109/L. Bone marrow was hypercellular with increase of megakaryocytes (Fig. 1) and without increase of blasts (0.4%). Chromosomal analysis of bone marrow cells presented normal karyotype, and genetic tests revealed the presence of JAK2 V617F mutation. She was diagnosed with ET. Thrombocytosis had been well controlled by the administration of hydroxyurea; 2 years after initial diagnosis, her routine blood examination showed leukocytosis (white blood cells 14.0 × 109/L with 82% of blasts), anemia (hemoglobin 91 g/L), and thrombocytopenia (platelets 24 × 109/L). Bone marrow was hypercellular and infiltrated with 80% blasts, some of which possessed Auer rods (Fig. 2A). These blasts were positive for myeloperoxidase staining (Fig. 2B) and presented positivity for CD13, CD19, CD34, and CD56 on flow cytometry. G-banded chromosome analysis of bone marrow cells showed 46,XX,t(8;21)(q22;q22.1)[4]/45,idem,-X[16]. RUNX1::RUNX1T1 chimeric transcript was confirmed by reverse transcriptase polymerase chain reaction analysis. BCR::ABL1 fusion was not detected. She was diagnosed with AML with t(8;21)(q22;q22.1); RUNX1::RUNX1T1.
A Morphologic features of blasts in bone marrow at the leukemic transformation (May–Giemsa staining, original magnification ×1000). Blasts contained fine azurophilic granules in abundant basophilic cytoplasm; arrow shows a blast with a single and sharp Auer rod. B Blasts were positive for myeloperoxidase staining
Allogeneic hematopoietic transplantation therapy was discussed, but she was considered to be transplant-ineligible because of higher age. Single usage of JAK inhibitor (ruxolitinib) was also clinically impractical as its efficacy in this setting was not established. As hypomethylating agent therapy was increasingly recognized as an alternative to induction chemotherapy for post-MPN AML [5, 17], she was treated with combination chemotherapy by venetoclax and azacytidine. After the first cycle of the therapy, blasts disappeared from peripheral blood and decreased to 1.4% in bone marrow. During the course of the second cycle, she developed sepsis and pneumonia accompanying severe cardiac and respiratory failure. After the recovery from infections, she chose to be treated with best supportive care and the chemotherapy was discontinued.
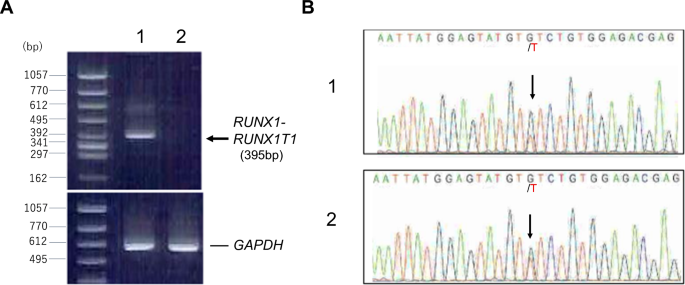
For clonality analysis, RNA was prepared from CD34-sorted circulating leukemic blasts before chemotherapy and peripheral leukocytes after chemotherapy (65 days after the start of the therapy), consisting of 97% neutrophils, and subjected to reverse transcriptase polymerase chain reaction and nucleotide sequence analysis. As shown in Fig. 3, RUNX1::RUNX1T1 chimeric transcript disappeared after chemotherapy, whereas heterozygous mutation of JAK2 V617F was present in both samples, indicating that the leukemic blasts were derived from JAK2-mutated ET clone. Mutation of KIT or FLT-3 was not detected in leukemic blasts.
A RT-PCR detecting RUNX1::RUNX1T1 chimeric transcript. The sequences of primers were as follows; RUNX1 (forward), 5′- CTACCGCAGCCATGAAGAACC -3′; RUNX1T1 (reverse), 5′- AGAGGAAGGCCCATTGCTGAA -3′. Lane 1; leukemic blasts before chemotherapy, lane 2; peripheral leukocytes after chemotherapy. B Sequence analysis showing JAK2 V617F mutation. cDNA was amplified using primers, 5′-ATTTTTAAAGGCGTACGAAGAGAAGTAG-3′ (forward) and 5′-ATAAGCAGAATATTTTTGGCACATACAT-3′ (reverse). PCR product containing the reported mutation point was directly sequenced. Arrows indicate G > T substitution. 1; leukemic blasts before chemotherapy, 2; peripheral leukocytes after chemotherapy
Discussion and conclusion
Recent studies revealed that secondary AMLs evolving from Ph-negative MPNs present unique cytogenetic and molecular features distinct from de novo AML, including the higher frequency of somatic mutations in TP53, ASXL1, IDH1/2, TET2, EZH2, and RUNX1 genes and the lower frequency of those in FLT3, MPM1, CEBPA, and WT1 [3, 4]. Chromosomal alterations related to poor-risk outcomes in MPNs include complex karyotypes, inv(3)(q21.3q26.2)/t(3;3)(q21.3;q26.2), i(17)(q10), -7/7q-, 12p-/12p11.2, or 11q23 rearrangements [5].
On the contrary, balanced chromosomal translocations frequently observed in de novo AML with good-risk complex are quite rare in post-MPN AML; 11 cases of acute promyelocytic leukemia with t(15;17)(q22;q11-12); PML::RARA evolved from MPNs were reported so far [6,7,8,9,10,11,12,13,14,15]. However, chromosomal rearrangements involving core binding factor genes, t(8;21) (q22;q22.1); RUNX1::RUNX1T1 or inv(16)(p13.1q22)/t(16;16)(p13.1;q22); CBFB::MYH11 were not reported, except for one case with ET-derived AML presenting t(8;21), in whom other gene mutations was not investigated [16]. Thus, to our best knowledge, the present case is the first one with JAK2 mutation preceding the acquisition of t(8;21).
Coexistence of JAK2 V617F mutation and t(8;21) chromosomal translocation is a rare but recurrent combination of genetic aberration in AML. JAK2 mutation has been reported to occur in a small number of de novo AML, with a relatively high incidence in subtype with t(8;21) associated with unfavorable clinical outcomes [18,19,20]. In these cases, JAK2 mutation was recognized as an additional genetic event after the formation of.RUNX1::RUNX1T1 chimeric gene. In our case, the opposite order of events, JAK2 mutation preceding the acquisition of t(8;21) was postulated, indicating that the occurrence of t(8;21) can be a late event in the progression of MPN, similarly as in a few cases with chronic myeloid leukemia with occurrent t(8;21) translocation and Ph chromosome [21,22,23,24,25,26].
Although high frequency of erythroblastic and megakaryoblastic phenotype was reported in post-MPN AML [27], our case presented typical morphological and immunophenotypic features associated with t(8;21) AML, such as the emergence of Auer rod and the expression of CD19, CD34, and CD56 [28]. The findings indicated that late-appearing cytogenetic abnormality would also define the phenotype of secondary AML from MPN.
Hydroxyurea, the most common cytoreductive agent employed in the management of MPN, interferes with DNA synthesis and may have mutagenic and leukemogenic potential. However, the recent study indicated that the use of hydroxyurea does not increase the risk of secondary malignancies, including AML and myelodysplastic syndrome [29]. Thus, it remains undetermined whether hydroxyurea promoted the formation of RUNX1::RUNX1T1 chimeric gene leading to leukemic transformation or not in this case.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- MPN:
-
Myeloproliferative neoplasm
- AML:
-
Acute myeloid leukemia
- ET:
-
Essential thrombocythemia
- Ph:
-
Philadelphia
References
Tefferi A, Pardanani A. Myeloproliferative neoplasms: a contemporary review. JAMA Oncol. 2015;1:97–105.
Cerquozzi S, Tefferi A. Blast transformation and fibrotic progression in polycythemia vera and essential thrombocythemia: a literature review of incidence and risk factors. Blood Cancer J. 2015;5: e366. https://doi.org/10.1038/bcj.2015.95.
Dunbar AJ, Rampal RK, Levine R. Leukemia secondary to myeloproliferative neoplasms. Blood. 2020;136:61–70.
Luque Paz D, Jouanneau-Courville R, Riou J, et al. Leukemic evolution of polycythemia vera and essential thrombocythemia: genomic profiles predict time of transformation. Blood Adv. 2020;4:4887–97.
Tefferi A, Alkhateeb H, Gangat M. Blast phase myeloproliferative neoplasm: contemporary review and 2024 treatment algorithm. Blood Cancer J. 2023;13:108. https://doi.org/10.1038/s41408-023-00878-8.
Batlle M, Fernández-Avilés F, Ribera JM, et al. Acute promyelocytic leukemia in a patient with idiopatic myelofibrosis. Leukemia. 1999;13:492–4.
Mollee PN, Taylor KM, Williams B, et al. Long-term molecular remission in promyelocytic transformation of myeloproliferative disease. Leukemia. 1999;13:648–50.
Kajiguchi T, Simokawa T, Saito M, et al. Transformation of polycythemia vera to acute promyelocytic leukemia. Int J Hematol. 2000;72:520–1.
Sato N, Furukawa T, Masuko M, et al. Acute promyelocytic leukemia developing in untreated essential thrombocythemia. Am J Hematol. 2002;71:114–6.
Braun TP, Maxson JE, Agarwal A, et al. Acute promyelocytic leukemia with JAK2 V617F and severe differentiation syndrome. Leuk Res Rep. 2015;4:8–11.
Mamorska-Dyga A, Wu J, Khattar P, et al. Acute promyelocytic leukemia co-existing with JAK2 V617F positive myeloproliferative neoplasm: a case report. Stem Cell Investig. 2016;3:8.
Morsia E, Goteri G, Torre E, et al. Acute promyelocyte leukemia arose from CALR 1 mutated post essential thrombocythemia-myelofibrosis with splanchnic vein thrombosis: a case report. Leuk Res Rep. 2021;15: 100243.
Nadiminti K, Silverman M, Bhagavathi S, et al. t(15;17) associated with primary myelofibrosis: a case report of an unusual clinical presentation and diagnostic dilemma. Onco Targets Ther. 2019;12:5449–55.
Li W-W, Sui X-F, Fun S, et al. Transformation from polycythemia vera to acute promyelocytic leukemia: case report and literature review. Medicine. 2022;101: e30064. https://doi.org/10.1097/MD.0000000000030064.
Zhang R, Liu R, Song H, et al. Clonal evolution analysis of a rare acute promyelocytic leukemia patient transforming from essential thrombocythemia. Ann Hematol. 2023;102:981–4.
Knottenbelt E, Hallett J, Jacobs P. 8;21 translocation in myelodysplasia secondary to essential thrombocythemia. Am J Hematol. 1989;30:233–5.
Gangat N, Guglielmelli P, Szuber N, et al. Venetoclax with azacytidine or decitabine in blast-phase myeloproliferative neoplasm: a multicenter series of 32 consecutive case. Am J Hematol. 2021;96:781–9.
Krauth M-T, Eder C, Alpermann T, et al. High number of additional genetic lesions in acute myeloid leukemia with t(8;21)/RUNX1-RUNX1T1: frequency and impact on clinical outcome. Leukemia. 2014;28:1449–58.
Iwanaga E, Nanri T, Matsuno N, Kawakita T, Mitsuya H, Asou N. A JAK2-V617F activating mutation in addition to KIT and FLT3 mutations is associated with clinical outcome in patients with t(8;21)(q22;q22) acute myeloid leukemia. Haematologica. 2009;94:433–5.
Christen F, Hoyer K, Yoshida K, et al. Genomic landscape and clonal evolution of acute myeloid leukemia with t(8;21): an international study on 331 patients. Blood. 2019;133:1140–51.
Ferro MT, Steegman JL, Escribano L, et al. Ph-positive chronic myeloid leukemia with t(8;21)(q22;q22) in blastic crisis. Cancer Genet Cytogenet. 1992;58:96–9.
Kojima K, Yasukawa M, Ishimaru F, et al. Additional translocation (8;21)(q22;q22) in a patient with Philadelphia-positive chronic myelogenous leukaemia in the blastic phase. Br J Haematol. 1999;106:720–2.
Zhang Y, Liu Y, Liu X, et al. Co-existence of t(9;22) and t(8;21) in primary blast phase of chronic myelogenous leukemia: clinical experience and literature review. Int J Clin Exp Pathol. 2019;12:1811–5.
Ma C-C, Chai Y, Chen HL, et al. Clonal evolution of AML1-ETO coexisting with BCR-ABL and additional chromosome abnormalities in a blastic transformation of chronic myeloid leukemia. J Int Med Res. 2020. https://doi.org/10.1177/0300060520919237.
Gong J-Y, Zhang Z-H, Zhang W, et al. Coexistence of recurrent chromosomal abnormalities and the Philadelphia chromosome in acute and chronic myeloid leukemias: report of five cases and review of literature. Mol Cytogenet. 2020;13:34–42.
Morita K, Jabbour E, Ravandi F, et al. Clinical outcomes of patients with concurrent core binding factor rearrangement and Philadelphia chromosome. Clin Lymphoma Myeloma Leuk. 2021;21:338–44.
Abdulkarim K, Girodon F, Johansson P, et al. AML transformation in 56 patients with Ph- MPD in two well defined populations. Eur J Haematol. 2009;82:106–11.
Arber DA, Porwit A, Brunning RD, et al. Acute myeloid leukaemia with recurrent genetic abnormalities. In: Swedlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, editors., et al., World Health organization classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: Lyon IARC Press; 2017. p. 130–49.
Wang R, Shallis RM, Stempel JM, et al. Second malignancies among older patients with classical myeloproliferative neoplasms treated with hydroxyurea. Blood Adv. 2023;7:734–43.
Acknowledgements
We thank Dr. Yuichi Sugahara, Higashimatsuyama Municipal Hospital, for lending BM biopsy specimen at the diagnosis with ET.
Funding
There were no funding sources to support this study.
Author information
Authors and Affiliations
Contributions
CA wrote the manuscript and took care of the patient. TS, KS, IO, AO, RA, and YI edited the manuscript and took care of the patient. EK performed molecular analysis. YM and YT reviewed and edited the manuscript. YN wrote and edited the manuscript. All authors approved the final manuscript and consented to publish the manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The study was performed with prior approval of the Institutional Review Board and the Ethics Committee of the Saitama Medical University Hospital (the committee’s reference number: 12-089). Written informed consent for the study was obtained from the patient.
Consent for publication
Written informed consent was obtained from the patient’s legal guardian for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Asou, C., Sakamoto, T., Suzuki, K. et al. Transformation into acute myeloid leukemia with t(8;21)(q22;q22.1); RUNX1::RUNX1T1 from JAK2-mutated essential thrombocythemia: a case report. J Med Case Reports 18, 372 (2024). https://doi.org/10.1186/s13256-024-04691-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-024-04691-0